Solvent extraction is the process of separating compounds by utilizing their relative solubilities. Full PDF Package Download Full PDF Package.

Basic Steps To Fractional Distillation Understanding Distillation
And concentrated using fractional distillation.

. Glucose ethanol. Dry distillation is the heating of solid materials to produce gaseous products which may condense into liquids or solids. WALAS S Chemical Process Equipment Selection and Design.
933C Describe the production of ethanol by fermentation of carbohydrates in aqueous solution using yeast to provide enzymes. Fermentation is an anaerobic. 2 cracking The first step in fractional distillation is displacing the crude oil.
3 Use of appropriate apparatus and techniques for conducting and monitoring chemical reactions. Download Full PDF Package. Fractional distillation at atmospheric pressure can concentrate ethanol to 956 by weight 895 mole.
934C Explain how to obtain a concentrated solution of ethanol by fractional distillation of the fermentation mixture. The mixture of liquid and vapour fractions gets separated in the tower depending on their weight and boiling point boiling point is the temperature at which the liquid phase. Ethanol can be produced by fermentation.
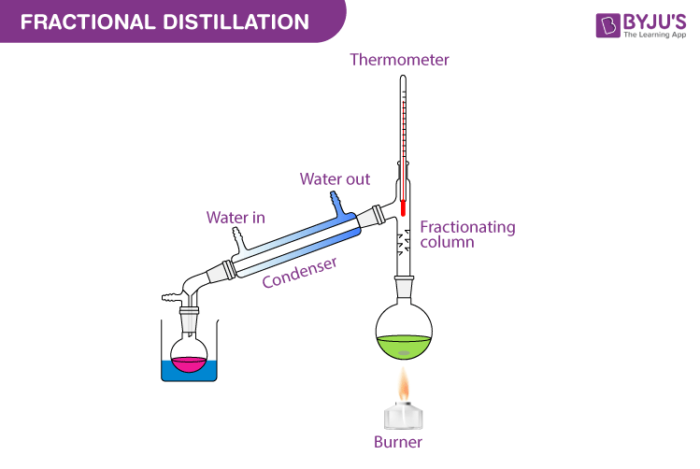
Explore the definition and process of solvent extraction and discover a. Decompose Page 16 of 32. The fractional distillation column separates the mixture into different compartments called fractions.
It is non-water miscible and has a flash point of approximately -45F varying with octane rating. List and describe several techniques used to separate mixtures. There exists a temperature gradient in the distillation tower where the top is cooler than the base.
Can be made from recycled paper or from trees. Crude oil is a finite resource. Fractional distillation is used to separate the compounds in crude oil.
Dry distillation may involve chemical changes such as destructive distillation or cracking and is. A short summary of this paper. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation.
Mass amount or quantity of matter Volume amount of space occupied ThereforeMATTER is anything that has mass and takes up space. Fractional distillation cracking and polymerisation all require a lot of energy. Gasoline has a vapor density between 3 and 4.
DEFINITION OF MATTER CHEMISTRY study of the composition structure and properties of matter and the changes it undergoes The two properties of MATTER are. Gasoline is a hydrocarbon produced from crude oil by fractional distillation. 24 Full PDFs related to this paper.
Therefore as with all products with a vapor density greater than 10 gasoline vapor s will seek low levels or remain. Distillation or classical distillation is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Making paper from trees requires.
Evaporating burn During fractional distillation the compounds condense at different temperatures.
Fractional Distillation Detailed Explanation Along With Diagrams

What Is Fractional Distillation Definition Process Video Lesson Transcript Study Com
0 Comments